|
Daynotes
Journal
Week of 3 March 2008
Latest
Update: Saturday, 8 March 2008
08:59 -0500 |
07:59
- Dinner last night with Paul and Mary.
I made a small bet with Barbara. I told her that when the food
arrived, I was going to announce, "Do you mind if I say grace?" Barbara
bet I'd get dumbfounded looks. I bet that one or the other, probably
Mary, would reply, "Only if you say it out loud." This is, after all, the woman who used the quote "We've done the impossible, and that makes us mighty" as the headline for her final BluePlanetRun.org blog entry.
Alas, Barbara won. When I asked Mary if she minded if I said grace, she replied, "Do you want to?" What was she thinking?
I'm getting into the home
forensics lab book. One of the fun things about writing a book like this
is figuring out how to make the procedures and technologies accessible
and affordable for home scientists.
For example, right now I'm stubbing out a lab
session on using chromatography to analyze drugs. Professional
forensics labs indeed use chromatography for this purpose, but
ordinarily they use TLC (thin-layer chromatography), GC (gas
chromatography), and HPLC (high performance/pressure liquid
chromatography). GC requires expensive equipment. LC (if not HPLC) can
be done in a home lab using a burette for the column, but requires substrates that are relatively
expensive and difficult to come by. Even TLC requires TLC plates, which
are available as far as I've been able to determine only in bulk
quantities much larger (and more expensive) than are suitable for home
labs.
My
first thought was to make TLC plates from microscope slides, using
albumen (egg white) or Elmer's Glue as the binder and cornstarch,
talcum powder, or crushed silica gel from drying packets as the
stationary phase. And that actually works pretty well. The problem is
that it's time-consuming and picky work, and I don't think the result
is worth the time required. So I decided to use simple paper
chromatography, which also works pretty well and is as useful to
illustrate the principles as the less accessible chromatography methods.
The
remaining problem is processing the chromatograms. Most home science
chromatography experiments use brightly-colored samples, such as food
coloring or inks, so no further processing is required once the
chromatograms are developed. For drugs, it's a bit more involved. For
example, I plan to separate aspirin, acetaminophen, ibuprofen,
naproxen, and caffeine, all of which are common components of OTC
painkillers (at least in the US; naproxen is still a prescription drug
in most of the world.)
All of those are colorless compounds, at
least in the concentrations present on a developed chromatogram. So I
need some means to process the developed chromatogram to make the
samples visible. I'll probably use UVA (a "black light" BWB tube) and
iodine fuming, but I need to do some experiments to verify that those
two methods will in fact reveal the samples.
Another
lab session from the same chapter is about identifying specific drugs
by examining their microcrystalline structure under the microscope.
Although white light is somewhat useful for this purpose, what's really
needed is polarized light. But if you check out the prices of
polarizing microscopes, you'll find they're out of reach for most home
scientists.
So, I searched Google for "polarizing film" and
found that Edmund Scientific sells 2" (5 cm) squares of polarizing
film, two for $7.50. I'll put one of those under the stage somewhere,
probably on top of the illuminator or under the condensor. That will be
the fixed filter. I'll mount the other piece of film in cardboard and
hold it between my eye and the ocular, rotating it as necessary.
Cool. I just came across a page that describes what looks very much like the high-speed camera
we used the summer before I left for college to measure how fast I
served a tennis ball. I was thinking the name of the camera was
something like Hulcher, but this Wollensack Fastax model looks a lot
like the camera we used, as best I can remember after nearly 40 years.
The text says this camera operated on AC, but I remember that the one
we used had a large, heavy battery pack. It also mentions a fixed frame
rate of 5,600 frames/second, while I seem to recall that the one we
used had variable speeds up through something like 5,000 or 10,000 frames/second.
I do remember the screeching noise, though.
08:07
- Here's a disturbing article
from Ed Foster's Gripelog. Apparently, most or all current televisions
are built with replaceable modules. The problem is, manufacturers
produce a very limited number of replacement modules during the
production run of the specific model and then stop producing the
modules. If your set fails out of warranty, chances are it's not
repairable. And it's very likely to fail early because nowadays, thanks
to EU regulators, essentially all consumer electronics are built using
lead-free solder, which fails early and often catastrophically due to
the growth of tin whiskers.
Not
that long ago, a television was
likely to give good service for a decade or more. Some units failed
earlier than that, of course, but they could be repaired. Nowadays,
because new modules are no longer available and old modules cannot be
repaired, the expected lifespan of a new television set is, what, maybe
four or five years? And when it fails your only option
is to scrap it and buy a new one, which will be no better than the one
you're replacing. Talk about a scam.
This is just one more
reason to avoid HDTV. We'll hang on to our 27" analog Panasonic until
it gives up the ghost. And we have a 25" unit down in the guest suite,
along with a 20" unit (with our only remaining VCR built-in). We could
continue watching TV and DVDs on them until they die. At that point, I
don't know what we'll do. It may be we'll just decide to stop watching
TV entirely.
08:25
- More on TVs dying young.
From: Christensen, Chris (Aspen Research)
To: Robert Bruce Thompson
Date: Tue Mar 4 11:56:45 2008
Re: Rohs whining
Robert:
From the Ed Foster article, excessive whining. First, the fluid in
electrolytic capacitors is not corrosive. It might be poisonous, or a
deadly carcinogen, but it has to be a material with a high and stable
dielectric constant, i.e., no charge carrying ion species, which
eliminates acids and caustics. It's in an aluminum can for pities sake.
The electrolytic caps I've failure analyzed used ethylene glycol for a
dielectric fluid. Big ones may have used polychlorinated biphenols (in
the past).
As
far as ROHS compliant (lead free, among other elements/compounds)
devices, there are issues, but I know that the disk drive manufacturers
(one to my personnel knowledge) have worked very hard on reliability of
lead free solder joints. I assume other computer part manufacturers are
working diligently to produce reliable devices. There's enormous
amounts of literature on succesful implementation of lead free solders.
As far as tin whisker formation, that was understood and solved by AMP
(tin plated terminals, static switches, etc..) more than 40 years ago.
I had the pleasure of being able to consult James Whitley (from AMP,
since deceased) back in the 80's about a tin contact problem.
My
opinion is that whining about ROHS and lead free solders is a way to
distract the end user from the fact that the devices in question are
outsourced to countries that don't bother to obtain reliable starting
materials (solders, fluxes, etc..), won't read the literature and
follow well established procedures to make reliable devices, and won't
accept responsibility for their own work. It doesn't matter whose name
is on the device if the work is outsourced to the lowest cost gypsy
producers that can be found.
Here's nine-inch nails in the coffin
of the music labels. Trent Reznor, the front-man for Nine Inch Nails,
decided to bypass the music labels entirely for their latest album.
Reznor "gets" the Internet, and in fact has admitted to using P2P
services to download music himself. The new album is available only
directly from the band, and they're offering several options,
from a free download to a $300 limited-edition deluxe set. That $300
deluxe set is limited to 2,500 copies, and they sold out within a day
of the announcement.
I confess that I know nothing about Nine
Inch Nails. As far as I know, I've never heard one of their tracks. But
I just finished downloading the nine free tracks, and when I have a
moment I'll give them a listen if only to support a band that really
gets the Internet. If I like it well enough to pay for it, I'll send
them the $5 to get the remaining 27 tracks. Which is the whole idea.
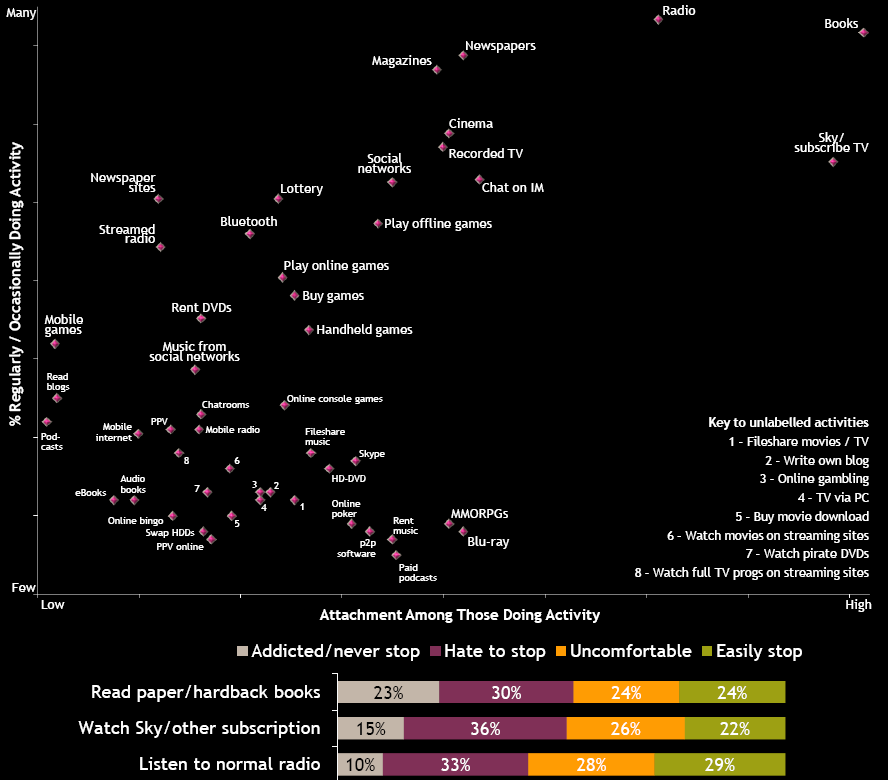
While I was munching around yesterday, I came across the Digital Entertainment Survey 2008
(PDF). It's pretty comprehensive. Although it's based on survey data
from the UK, I suspect much of what it concludes is also pertinent for
the US. I found this chart fascinating. Notice what's at the upper
right of the chart. Reading books is, except for listening to the
radio, the most popular activity, and the one that people are most
attached to. There may be hope yet.
08:45
- Here's some good news: FCC Okays Nudity On TV If It's Alyson Hannigan. Thanks to Brian Bilbrey for the link.
Right
now, I'm in research mode for the home forensics lab book, and I'm
learning a bunch of new stuff every day. A lot of the books I've bought
are old--anything from 1950 back to about 1890--because I need to find
out how things were done with wet chemistry back before instrumental
analysis methods were available. But I also have quite a few current
books, which I'm reading to get up-to-date with current practices.
I
was reading one of those yesterday to learn about current
state-of-the-art in forensic geology. I was surprised to learn that
density-gradient columns, although still in wide use for soil analysis,
are no longer considered a definitive forensic test.
Density-gradient
columns are simple conceptually. You start with two tall glass
cylinders and two liquids of different densities, most commonly
bromoform (density 2.89) and bromobenzene (density 1.499). You prepare
the two cylinders identically. For example, you might start by pouring
50 mL of bromoform into each cylinder. You then make up a solution of
45 mL of bromoform and 5 mL of bromobenzene, which has a density
slightly lower than pure bromoform. You carefully pour 50 mL of this
solution into each cylinder, being careful not to mix the second
solution with the first. The goal is to end up with two layers in each
cylinder. Then you make up a solution of 40 mL of bromoform with 10 mL
of bromobenzene, and add it as a third layer to each cylinder. You
repeat that process with 50 mL each of bromoform/bromobenzene solutions
mixed 35/15, 30/20, 25/25, 20/30, 15/35, 10/40, 5/45, and finally pure
bromobenzene.
You then allow the two cylinders to sit
undisturbed overnight, or sometimes for 24 to 48 hours. During that
time, the solutions blend and you end up with cylinders full of liquid
whose density varies smoothly from 1.499 at the top to 2.89 at the
bottom. You then prepare the unknown sample and a known sample for
comparison by drying them and sifting them to obtain subsamples with
similar particle sizes. You add equal masses of the unknown to one
cylinder and the comparison known to the other cylinder and allow the
samples to settle.
Once the samples finish settling, you end up
with two cylinders full of fluid with horizontal striations where the
different components of each sample have achieved density equilibrium,
essentially providing a "fingerprint" of each sample.
This all
sounds very scientific, and frankly I don't see anything at all wrong
with it conceptually. People have been convicted of major crimes based
solely on density-gradient column evidence, and even now some forensic
scientists testify to such evidence in court. The problem is, the
density-gradient column method is not definitive but at most
suggestive. No less an authority than the world-renowned forensic
geologist Raymond C. Murray has stated, "But some professionals still
emphasize the advantages of the density-gradient column method: you can
train any idiot to do it, it produces pretty pictures for a jury, and
nobody has to think."
07:59
- I ordered a microscope from Home Science Tools yesterday. The model I decided on is the National Optical 161,
which is a dual-head microscope. I need the second head for shooting
images. I already have a microscope adapter for our Pentax DSLRs, so it
looks like I'll be learning something about photomicroscopy.
After talking it over with Mike, my contact at National
Optical, I ordered the 161-ASC model, which has upgraded objectives. I also ordered the 965-160 eyepiece reticle.
Why
National Optical? Obviously, if my budget was unlimited, I'd have
bought a German or Japanese scope from Leitz, Zeiss, Nikon, Olympus, or
one of the other top brand-name manufacturers. Unfortunately, prices
for those scopes start in the low- to mid-four figures and rapidly head
up into five figures. That not's practical for me. Even more important,
it's not practical for my readers.
I needed a microscope that
offered good optical and mechanical quality at a reasonable price, and nowadays that means
buying a Chinese scope. As is generally true of Chinese products,
Chinese microscopes range in quality from quite good to incredibly poor. I did some
checking around and found that National Optical has a reputation for
offering the best Chinese microscopes available, and at reasonable
prices. The model I ordered, for example, retails for about $725 and
has a street price about $100 lower. That's not inexpensive by most
people's reckoning, but it sure beats the $2,000 or more that a similar
German or Japanese model would cost.
And this is actually more microscope than my readers will need. When I told Mike that the
market for the book was middle- and high-school students and home
science enthusiasts, he pointed out that the Model 161 is a
university-level scope, and more than my readers would really need,
even without the upgraded objectives. It was when I pointed out that I
needed to shoot images good enough to be published in a book that Mike
suggested going with the upgraded objectives, because photography is
much less forgiving than visual work.
08:59
- I got the first of the QC2 galley proof PDFs yesterday. I've posted the Preface and chapters 1 through 3 on the subscribers' page.
Others will follow as I receive them. There are some minor changes to
be made, but these are pretty much what the printed book will look
like. If you take a look at them, please let me know if you see any
problems. (I've already told me editors about the hanging paragraph
problem in the volumetric glassware section in chapter 3, so you
needn't point that one out.)
We're getting very close now.
There'll be only minor corrections to the galley proofs, after which
the book will go to the printers. As far as I know, the projected date
for availability is still 1 April, although that's really pushing it,
particularly for a 4-color book. My guess is that it'll be more like 1
May, but we'll know soon enough.
00:00
-
Copyright
© 1998,
1999, 2000, 2001, 2002, 2003, 2004, 2005, 2006, 2007, 2008 by Robert
Bruce
Thompson. All
Rights Reserved.